Table Of Content

The treatment may be drugs, devices, or procedures studied for diagnostic, therapeutic, or prophylactic effectiveness. Control measures include placebos, active medicines, no-treatment, dosage forms and regimens, historical comparisons, etc. When randomization using mathematical techniques, such as the use of a random numbers table, is employed to assign patients to test or control treatments, the trials are characterized as Randomized Controlled Trials. Non-randomized trials are interventional study designs that compare a group where an intervention was performed with a group where there was no intervention. These are convenient study designs that are most often performed prospectively and can suggest possible relationships between the intervention and the outcome. However, these study designs are often subject to many types of bias and error and are not considered a strong study design.
Research methods
Sometimes epidemiologists and others will refer to these as more measures of association rather than separating them into their own category. Because they are very interrelated, it does not matter whether you refer to them as measures of effect or measures of association but rather when and how to use them. When we’re focused on population health, looking at relative differences like the odds ratio or relative risk is extremely useful to decide where we want to make a difference and what factors we should spend our time and energy on.
Longitudinal vs Transversal Studies
Randomized trials can also be classified as quantitative and comparative studies because research outcomes can be measured and compared easily. Clinical/experimental studies are performed in primary research, whereas secondary research consolidates available studies as reviews, systematic reviews and meta-analyses. Three main areas in primary research are basic medical research, clinical research and epidemiological research [Figure 2]. In almost all studies, at least one independent variable is varied, whereas the effects on the dependent variables are investigated.
Conducting an Experiment in Psychology - Verywell Mind
Conducting an Experiment in Psychology.
Posted: Mon, 30 Oct 2023 07:00:00 GMT [source]
5.2 Relative Risk
A Pew Research Center experiment with one of its routinely asked values questions illustrates the difference that question format can make. Not only does the forced choice format yield a very different result overall from the agree-disagree format, but the pattern of answers between respondents with more or less formal education also tends to be very different. Because of concerns about the effects of category order on responses to closed-ended questions, many sets of response options in Pew Research Center’s surveys are programmed to be randomized to ensure that the options are not asked in the same order for each respondent. Rotating or randomizing means that questions or items in a list are not asked in the same order to each respondent.
Understanding common key indicators of successful and unsuccessful cancer drug trials using a contrast mining ... - ScienceDirect.com
Understanding common key indicators of successful and unsuccessful cancer drug trials using a contrast mining ....
Posted: Thu, 16 Feb 2023 21:19:33 GMT [source]
The response variable (whether or not the respondent had SMND) is assessed when the study begins, and whether or not subjects had exposure to metals (explanatory variable) is determined from the past.This observational study has a backward direction. Cross-sectional studies are another wonderful way to obtain initial information about diseases within a community, including mortality rates and associations between exposure and risks. Data is collected at a single point in time, which provides a snapshot of a disease. One of the main advantages of this design is that when a case appears in the cohort, controls who were at risk at the time can be selected. This tactic gives researchers control over any confounding effects that may be established. As mentioned above, nested case-control studies reduce the costs of following up a whole cohort.
Subjective Outcomes: Questionnaires and Data Forms
By implementing Intervention II among all NCAA women’s basketball players, we would reduce the total burden of noncontact ACL injuries in this population by less than 1 percent. This intervention may work well on an individual level but not as a population level intervention for noncontact ACL injuries. The intervention reduces the risk of noncontact ACL injury 3 percent compared to baseline. Athletes who participate in Intervention II have 0.38 times the risk of a noncontact ACL injury compared to athletes at baseline.
Retrospective and prospective cohort study design
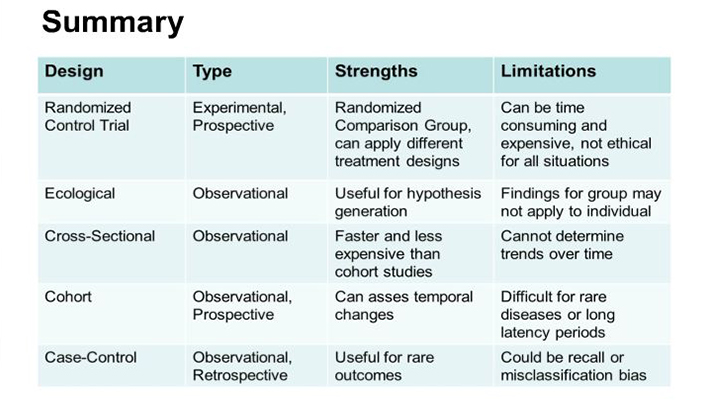
Studies were classified based on the method of collection and evaluation of data. Clinical study methodology now needs to comply to strict ethical, moral, truth, and transparency standards, ensuring that no conflict of interest is involved. A medical research pyramid has been designed to grade the quality of evidence and help physicians determine the value of the research. Randomised controlled trials (RCTs) have become gold standards for quality research. EBM now scales systemic reviews and meta-analyses at a level higher than RCTs to overcome deficiencies in the randomised trials due to errors in methodology and analyses. Controlled Clinical Trials - Clinical trials involving one or more test treatments, at least one control treatment, specified outcome measures for evaluating the studied intervention, and a bias-free method for assigning patients to the test treatment.
The analytic methods of pre-post studies depend on the outcome being measured. If there are multiple treatment arms, it is also likely that the difference from beginning to end within each treatment arm are analysed. A study that examines the relationship between diseases (or other health-related characteristics) and other variables of interest as they exist in a defined population at one particular time (ie exposure and outcomes are both measured at the same time). Best for quantifying the prevalence of a disease or risk factor, and for quantifying the accuracy of a diagnostic test. In analytic observational studies, the researcher simply measures the exposure or treatments of the groups. Analytical observational studies include case””control studies, cohort studies and some population (cross-sectional) studies.
We make every effort to find cases that occurred earlier than when we first realized something might be amiss. In figure 3.16, we see an example of a line listing from an anthrax outbreak. Each row corresponds to a different case, and we include all the possible details relevant to the case status and demographic information.
Prevention can include changes to protective equipment, engineering controls, management, policy or any element that should be evaluated as to a potential cause of disease or injury. This study design compares clusters of people, usually grouped based on their geographical location or temporal associations (1,2,6,9). Ecological studies assign one exposure level for each distinct group and can provide a rough estimation of prevalence of disease within a population. An example of an ecological study is the comparison of the prevalence of obesity in the United States and France. There are inherent potential weaknesses with this approach, including loss of data resolution and potential misclassification (10,11,13,18,19). Typically these studies derive their data from large databases that are created for purposes other than research, which may introduce error or misclassification (10,11).
The details of how the data will be collected (Chap. 10) and ethical issues (Chap. 4) must also be considered.Furthermore, the limitations of the study must be communicated (Chap. 9). The advantages and disadvantages of each study type are discussed later (Sect. 9.2), after these study types are discussed in greater detail in the following chapters. Methodological studies can help experts test if the employed research instrument can be interchanged with another one. Therefore, studies that assess repeatability and agreement of a research instrument are crucial. In the end, repeatability and avoiding bias are important aspects of research.
Example 3.13 (Backwards study) Pamphlett (2012) examined patients with and without sporadic motor neurone disease (SMND), and asked about past exposure to metals. Research studies are sometimes described as 'prospective' or 'retrospective', but these terms can be misleading (Ranganathan and Aggarwal 2018) and their use not recommended (Vandenbroucke et al. 2014). Definition 3.2 (Observational study) Observational studies study relationships without an intervention. Therefore, a larger number of controls can be enrolled for each case to improve the statistical efficiency of the study.
The purpose of research design is to plan and structure a research study in a way that enables the researcher to achieve the desired research goals with accuracy, validity, and reliability. Research design is the blueprint or the framework for conducting a study that outlines the methods, procedures, techniques, and tools for data collection and analysis. Case study research design is used to investigate a single case or a small number of cases in depth. It involves collecting data through various methods, such as interviews, observations, and document analysis. The aim of case study research is to provide an in-depth understanding of a particular case or situation.