Table Of Content

All examples in this book use the version showing exposure in rows and outcome in columns. Case-control studies are used to find out whether a particular exposure could have been the source or cause of a disease, particularly in urgent health situations. We start by identifying who already has the disease (cases), then we find a set of people who are like the cases in every respect except they do not have disease. Because we start with people who are diseased, case-control studies are great when you are interested in studying people who have rare diseases.
Observational versus interventional (or experimental) studies
Remember that cohort studies are to identify the risk of disease in the exposed compared to the risk of disease in the unexposed. Medical research has evolved, from individual expert described opinions and techniques, to scientifically designed methodology-based studies. Evidence-based medicine (EBM) was established to re-evaluate medical facts and remove various myths in clinical practice.
Understanding Research Study Designs
Sham therapy is a comparison procedure or treatment which is identical to the investigational intervention except it omits a key therapeutic element, thus rendering the treatment ineffective. An example is a sham cortisone injection, where saline solution of the same volume is injected instead of cortisone. This helps ensure that patients do not know if they are receiving the active or control treatment. The process of blinding is utilized to help ensure equal treatment of the different groups, therefore continuing to isolate the difference in outcome between groups to only the intervention being administered (28–31).
U.S. Surveys
RCTs have conflicting interests and thus must be evaluated with careful scrutiny. EBM thus should not be restricted to RCTs and meta-analyses but must involve tracking down the best external evidence to answer our clinical questions. Expert opinion, experience, and authoritarian judgement were the norm in clinical medical practice. At scientific meetings, one often heard senior professionals emphatically expressing ‘In my experience,…… what I have said is correct! Practice gets outdated, if not updated with current evidence, depriving the clientele of the best available therapy.
5.2 Relative Risk
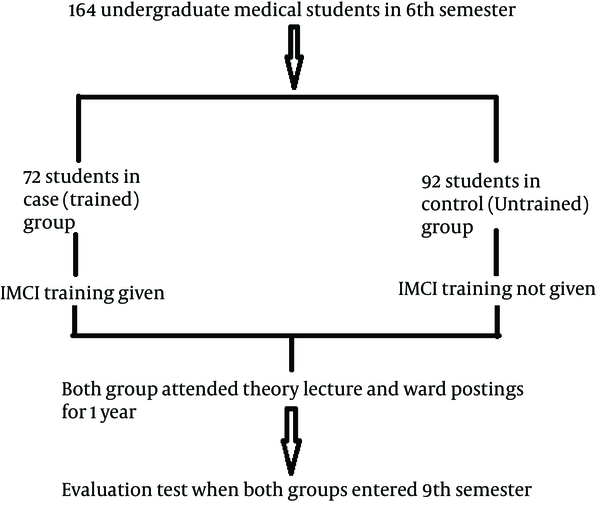
The most important part of steps 1 and 2 is that we must verify that the diagnosis we think is the problem is in fact the correct diagnosis. For example, if we think that we are having an outbreak of meningitis A, we should confirm that all of the people who are sick actually have meningitis A. It is usually called a meta-analysis by the author or sponsoring body and should be differentiated from reviews of literature. Matched-Pair Analysis - A type of analysis in which subjects in a study group and a comparison group are made comparable with respect to extraneous factors by individually pairing study subjects with the comparison group subjects (e.g., age-matched controls). Compliance is the degree of how well study participants adhere to the prescribed intervention.
Literature Reviews: Types of Clinical Study Designs
An analytic study attempts to quantify the relationship between two factors, that is, the effect of an intervention (I) or exposure (E) on an outcome (O). To quantify the effect we will need to know the rate of outcomes in a comparison (C) group as well as the intervention or exposed group. Whether the researcher actively changes a factor or imposes uses an intervention determines whether the study is considered to be observational (passive involvement of researcher), or experimental (active involvement of researcher).
The conclusions derived from a research study can either improve health care or result in inadvertent harm to patients. Hence, this requires a well‐designed clinical research study that rests on a strong foundation of a detailed methodology and governed by ethical clinical principles. The purpose of this review is to provide the readers an overview of the basic study designs and its applicability in clinical research. Systematic reviews can be used to summarize the results of all available medical studies and controlled trials. In fact, they can provide vital information about the effectiveness of an intervention.
The next generation of evidence-based medicine - Nature.com
The next generation of evidence-based medicine.
Posted: Mon, 16 Jan 2023 08:00:00 GMT [source]
In this study design subtype, the source of controls is usually adopted from the past, such as from medical records and published literature.1 The advantages of this study design include being cost‐effective, time saving and easily accessible. However, since this design depends on already collected data from different sources, the information obtained may not be accurate, reliable, lack uniformity and/or completeness as well. Though historically controlled studies maybe easier to conduct, the disadvantages will need to be taken into account while designing a study. The experimental study design can be classified into 2 groups, that is, controlled (with comparison) and uncontrolled (without comparison).1 In the group without controls, the outcome is directly attributed to the treatment received in one group. This fails to prove if the outcome was truly due to the intervention implemented or due to chance. This can be avoided if a controlled study design is chosen which includes a group that does not receive the intervention (control group) and a group that receives the intervention (intervention/experiment group), and therefore provide a more accurate and valid conclusion.
In simple randomization, the subjects are randomly allocated to experiment/intervention groups based on a constant probability. That is, if there are two groups A and B, the subject has a 0.5 probability of being allocated to either group. This can be performed in multiple ways, and one of which being as simple as a ‘flip of a coin’ to using random tables or numbers.17 The advantage of using this methodology is that it eliminates selection bias. However, the disadvantage with this methodology is that an imbalance in the number allocated to each group as well as the prognostic factors between groups. Clinical trials are also known as therapeutic trials, which involve subjects with disease and are placed in different treatment groups. One of the earliest clinical trial studies was performed by James Lind et al in 1747 on sailors with scurvy.12 Lind divided twelve scorbutic sailors into six groups of two.
The primary measure of association that is calculated in a cohort study is the relative risk (the risk or incidence of the outcome in the exposed compared to the risk or incidence of the outcome in the unexposed). There are two primary categories of study designs (figure 3.1), and the primary difference between the two is whether or not we control the study factors. Cross-Sectional Studies - Studies in which the presence or absence of disease or other health-related variables are determined in each member of the study population or in a representative sample at one particular time. This contrasts with LONGITUDINAL STUDIES which are followed over a period of time. There are several types of research study designs, each with its inherent strengths and flaws.
In a case report, the researcher describes his/her experience with symptoms, signs, diagnosis, or treatment of a patient. Sometimes, a group of patients having a similar experience may be grouped to form a case series. Observational studies are those where the researcher is documenting a naturally occurring relationship between the exposure and the outcome that he/she is studying. The researcher does not do any active intervention in any individual, and the exposure has already been decided naturally or by some other factor. For example, looking at the incidence of lung cancer in smokers versus nonsmokers, or comparing the antenatal dietary habits of mothers with normal and low-birth babies. In these studies, the investigator did not play any role in determining the smoking or dietary habit in individuals.
This in turn poses the question for a new disease entity and further queries the investigator to look into mechanistic investigative opportunities to further explore. However, in a case series, the cases are not compared to subjects without the manifestations and therefore it cannot determine which factors in the description are unique to the new disease entity. However, a big disadvantage is that, since exposures and outcomes are measured at the same time, researchers can’t determine which comes first. Note that a response over 80% is needed to avoid bias and improve generalizability. In general, smaller samples with high response rates are more useful than larger samples with low response rates. Badawi and colleagues (1998) designed a case-control study to measure risk factors for newborn encephalopathy.
In retrospective studies, the outcome of interest has already occurred (or not occurred – e.g., in controls) in each individual by the time s/he is enrolled, and the data are collected either from records or by asking participants to recall exposures. By contrast, in prospective studies, the outcome (and sometimes even the exposure or intervention) has not occurred when the study starts and participants are followed up over a period of time to determine the occurrence of outcomes. Typically, most cohort studies are prospective studies (though there may be retrospective cohorts), whereas case–control studies are retrospective studies. An interventional study has to be, by definition, a prospective study since the investigator determines the exposure for each study participant and then follows them to observe outcomes. From an epidemiological standpoint, there are two major types of clinical study designs, observational and experimental.3 Observational studies are hypothesis‐generating studies, and they can be further divided into descriptive and analytic. Descriptive observational studies provide a description of the exposure and/or the outcome, and analytic observational studies provide a measurement of the association between the exposure and the outcome.
We often write two versions of a question and ask half of the survey sample one version of the question and the other half the second version. Respondents are assigned randomly to receive either form, so we can assume that the two groups of respondents are essentially identical. On questions where two versions are used, significant differences in the answers between the two forms tell us that the difference is a result of the way we worded the two versions. In addition to the number and choice of response options offered, the order of answer categories can influence how people respond to closed-ended questions. Research suggests that in telephone surveys respondents more frequently choose items heard later in a list (a “recency effect”), and in self-administered surveys, they tend to choose items at the top of the list (a “primacy” effect).
Interventional studies are experiments where the researcher actively performs an intervention in some or all members of a group of participants. This intervention could take many forms – for example, administration of a drug or vaccine, performance of a diagnostic or therapeutic procedure, and introduction of an educational tool. For example, a study could randomly assign persons to receive aspirin or placebo for a specific duration and assess the effect on the risk of developing cerebrovascular events. A variable represents a measurable attribute that varies across study units, for example, individual participants in a study, or at times even when measured in an individual person over time. Some examples of variables include age, sex, weight, height, health status, alive/dead, diseased/healthy, annual income, smoking yes/no, and treated/untreated.
Compliance or non-compliance to the intervention can have a significant impact on the results of the study (26–29). If there is a differentiation in the compliance between intervention arms, that differential can mask true differences, or erroneously conclude that there are differences between the groups when one does not exist. The measurement of compliance in studies addresses the potential for differences observed in intervention arms due to intervention adherence, and can allow for partial control of differences either through post hoc stratification or statistical adjustment. Observational study design measures of disease, measures of risk, and temporality. Each type of study has different methods with unique advantages and disadvantages.

No comments:
Post a Comment